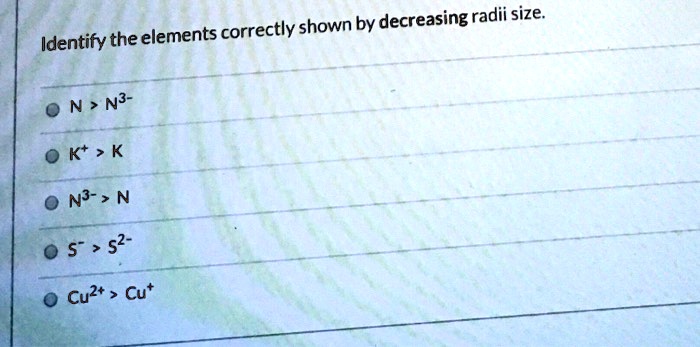
Identify the elements correctly shown by decreasing radii size – Delving into the realm of atomic and elemental properties, this exploration unravels the intriguing concept of decreasing radii size, a fundamental characteristic that governs the arrangement and behavior of elements within the periodic table. By embarking on this journey, we will uncover the factors influencing radii size, trace its trends across periods, and delve into the exceptions that challenge these patterns.
Furthermore, we will illuminate the practical applications of radii size data, showcasing its significance in predicting chemical properties, determining crystal structures, and advancing materials science and nanotechnology.
1. Introduction

The radius of an atom, often referred to as the atomic radius, is a measure of the distance from the nucleus to the outermost electron shell. Radii size plays a crucial role in determining the chemical and physical properties of elements.
The concept of decreasing radii size across periods in the periodic table is a fundamental principle in chemistry.
The periodic table is an organized arrangement of elements based on their atomic number, electron configuration, and recurring chemical properties. As we move from left to right across a period (row) in the periodic table, the atomic number increases. This increase in atomic number corresponds to an increase in the number of protons and electrons in the atom.
However, the number of energy levels (shells) remains the same.
As more electrons are added to the same energy level, they experience an increased attraction to the positively charged nucleus. This stronger attraction pulls the electrons closer to the nucleus, resulting in a decrease in the atomic radius.
2. Factors Influencing Radii Size

Atomic Number, Identify the elements correctly shown by decreasing radii size
The atomic number of an element is a primary factor that determines its atomic radius. As the atomic number increases, the number of protons in the nucleus increases. The increased positive charge of the nucleus exerts a stronger attractive force on the electrons, drawing them closer and reducing the atomic radius.
Electron Shielding
Electron shielding, also known as the shielding effect, refers to the ability of inner electrons to reduce the attractive force between the nucleus and the outermost electrons. Inner electrons form a shield or barrier between the nucleus and the outermost electrons, reducing the effective nuclear charge experienced by the outermost electrons.
This shielding effect weakens the attraction between the nucleus and the outermost electrons, resulting in a larger atomic radius.
Number of Energy Levels
The number of energy levels (shells) in an atom also influences its atomic radius. As the number of energy levels increases, the outermost electrons are located further from the nucleus. The increased distance between the nucleus and the outermost electrons reduces the attractive force, leading to a larger atomic radius.
3. Trends in Radii Size

General Trend
Across periods in the periodic table, there is a general trend of decreasing radii size from left to right. This trend is a direct consequence of the increasing atomic number and the corresponding increase in the number of electrons in the same energy level.
For example, consider the elements sodium (Na), magnesium (Mg), and aluminum (Al) in period 3. Sodium has an atomic number of 11, magnesium 12, and aluminum 13. As we move from sodium to magnesium to aluminum, the number of electrons in the outermost energy level remains the same (two), while the atomic number increases.
This increase in atomic number leads to a stronger attraction between the nucleus and the outermost electrons, resulting in a decrease in atomic radius.
Reasons for the Trend
The decrease in atomic radius across periods can be attributed to the following factors:
- Increased nuclear charge:As the atomic number increases, the number of protons in the nucleus increases, leading to a stronger attractive force between the nucleus and the electrons.
- Shielding effect:The shielding effect of inner electrons is less effective for outermost electrons, as they are located further from the nucleus. This reduced shielding results in a stronger attraction between the nucleus and the outermost electrons.
- Number of energy levels:The outermost electrons in a period occupy the same energy level. As the atomic number increases, the number of electrons in this energy level remains constant, while the number of protons in the nucleus increases. This leads to a decrease in the atomic radius.
4. Exceptions to the Trend: Identify The Elements Correctly Shown By Decreasing Radii Size
Deviations from the General Trend
While the general trend is a decrease in atomic radius across periods, there are some exceptions to this trend. Certain elements exhibit larger atomic radii than expected based on their position in the periodic table.
For example, beryllium (Be) has a larger atomic radius than boron (B), and nitrogen (N) has a larger atomic radius than oxygen (O). These deviations can be explained by considering the electronic configurations of these elements.
Reasons for Exceptions
- Incomplete electron shells:Elements with incomplete electron shells, such as beryllium and nitrogen, have a tendency to expand their electron clouds to achieve a more stable configuration. This expansion leads to a larger atomic radius.
- Lanthanide contraction:The lanthanide elements (atomic numbers 57-71) exhibit a gradual decrease in atomic radius across the series. This contraction is due to the poor shielding effect of the 4f electrons, which results in a stronger attraction between the nucleus and the outermost electrons.
5. Applications of Radii Size Data
Predicting Chemical Properties
Knowledge of atomic radii can be used to predict the chemical properties of elements. For example, elements with smaller atomic radii tend to be more electronegative (have a greater tendency to attract electrons) and form stronger bonds with other elements.
Determining Crystal Structures
Atomic radii play a crucial role in determining the crystal structures of elements. The size and arrangement of atoms in a crystal lattice are influenced by their atomic radii. For example, elements with similar atomic radii tend to form close-packed crystal structures, while elements with significantly different atomic radii may form more complex structures.
Materials Science and Nanotechnology
In materials science and nanotechnology, atomic radii data is essential for designing and manipulating materials at the atomic and molecular level. By controlling the atomic radii of the constituent elements, scientists can tailor the properties of materials for specific applications, such as semiconductors, catalysts, and energy storage devices.
Common Queries
What is the significance of decreasing radii size across periods in the periodic table?
Decreasing radii size across periods reflects the increasing effective nuclear charge experienced by electrons in higher periods. As the number of protons in the nucleus increases, the attractive force between the nucleus and electrons intensifies, drawing the electrons closer to the nucleus and reducing their radii.
How does electron shielding affect radii size?
Electron shielding refers to the ability of inner electrons to shield outer electrons from the attractive force of the nucleus. This shielding effect reduces the effective nuclear charge experienced by outer electrons, resulting in larger radii for elements with more inner electrons.
Can you provide an example of an element that deviates from the general trend of decreasing radii size?
Beryllium (Be) is an example of an element that deviates from the general trend of decreasing radii size. Despite having a higher atomic number than lithium (Li), beryllium has a slightly larger atomic radius due to the presence of a filled 2s orbital, which provides effective electron shielding for the outermost 2p electrons.