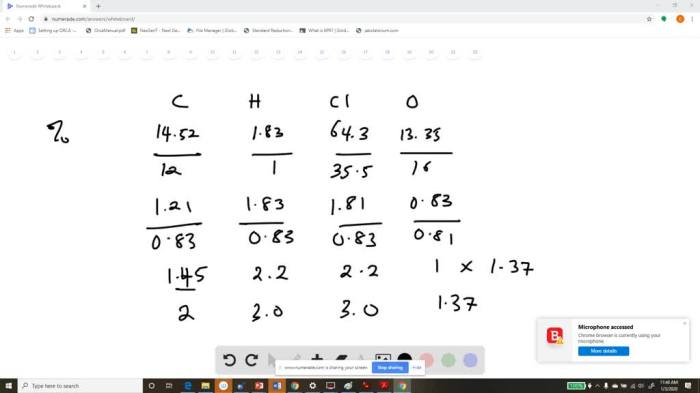
Draw the major organic product of the SN1 reaction, a fascinating chemical transformation that involves the substitution of a leaving group by a nucleophile via a carbocation intermediate. Delve into the intricacies of this reaction mechanism, exploring its factors, regio- and stereoselectivity, and practical applications.
The SN1 reaction, a cornerstone of organic chemistry, offers a unique perspective on the reactivity of organic compounds. By understanding the principles that govern this reaction, chemists can harness its power for diverse synthetic applications.
SN1 Reaction Mechanism
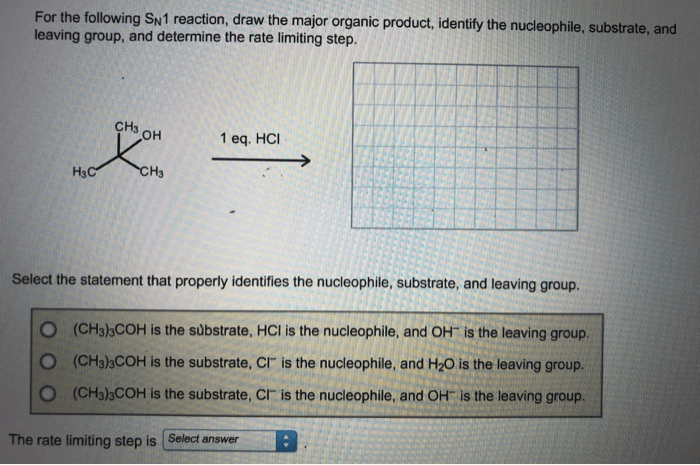
SN1 (Substitution Nucleophilic Unimolecular) reactions are a type of nucleophilic substitution reaction in which the rate-determining step is the formation of a carbocation intermediate. The mechanism involves the following steps:
- Ionization:The substrate undergoes ionization to form a carbocation and a leaving group.
- Nucleophilic attack:The carbocation intermediate is attacked by a nucleophile, resulting in the formation of the product.
Factors Affecting SN1 Reactions
The rate and selectivity of SN1 reactions are influenced by several factors:
- Substrate structure:Carbocations are more stable when they are formed from tertiary substrates than from secondary or primary substrates. This is because tertiary carbocations have more alkyl groups attached to the positively charged carbon, which helps to stabilize the charge.
- Solvent polarity:SN1 reactions are favored by non-polar solvents. This is because non-polar solvents do not solvate the ions involved in the reaction, which makes the ionization step more favorable.
- Nucleophile strength:Stronger nucleophiles are more likely to react with carbocations. This is because stronger nucleophiles are better able to donate electrons to the positively charged carbon.
Regioselectivity in SN1 Reactions
SN1 reactions are often regioselective, meaning that they occur preferentially at one site of the substrate. This regioselectivity is determined by the stability of the carbocation intermediate. The more stable the carbocation intermediate, the more likely it is to be formed and the more likely the reaction is to occur at that site.
Stereochemistry of SN1 Reactions
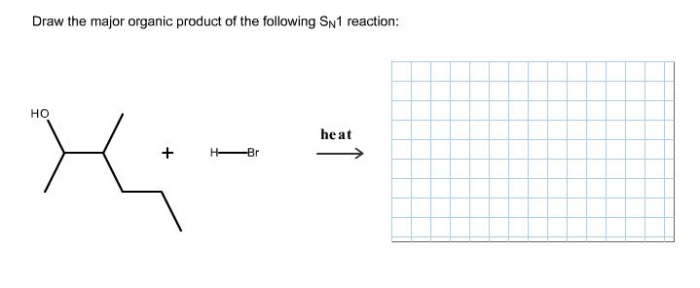
SN1 reactions can occur with either inversion or retention of configuration at the reaction center. The stereochemistry of the product is determined by the stereochemistry of the carbocation intermediate. If the carbocation intermediate is formed with inversion of configuration, then the product will also be formed with inversion of configuration.
If the carbocation intermediate is formed with retention of configuration, then the product will also be formed with retention of configuration.
Applications of SN1 Reactions
SN1 reactions are used in a variety of organic synthesis applications, including:
- Alkylation of alcohols:SN1 reactions can be used to alkylate alcohols to form ethers.
- Alkylation of amines:SN1 reactions can be used to alkylate amines to form tertiary amines.
- Rearrangements:SN1 reactions can be used to rearrange the carbon skeleton of organic molecules.
Comparison of SN1 and SN2 Reactions
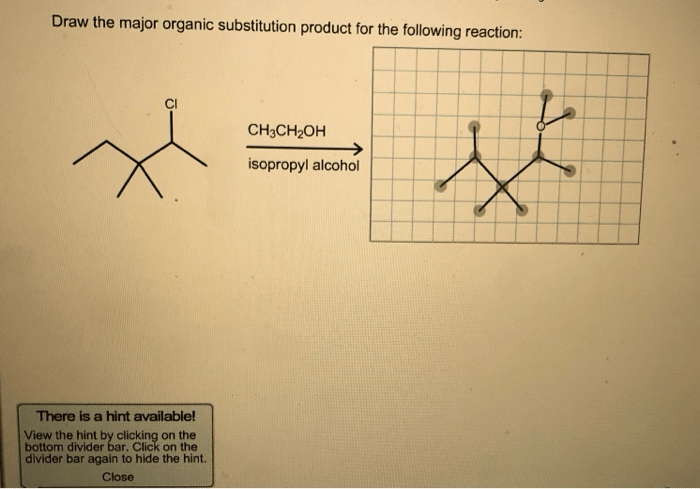
SN1 and SN2 reactions are two types of nucleophilic substitution reactions that differ in their mechanisms, factors affecting their rates, and regio- and stereoselectivity. The following table summarizes the key differences between the two reaction types:
| Characteristic | SN1 | SN2 |
|---|---|---|
| Mechanism | Unimolecular | Bimolecular |
| Rate-determining step | Formation of carbocation intermediate | Nucleophilic attack |
| Factors affecting rate | Substrate structure, solvent polarity, nucleophile strength | Substrate structure, nucleophile strength |
| Regioselectivity | Favored by tertiary substrates | Favored by primary substrates |
| Stereochemistry | Inversion or retention of configuration | Inversion of configuration |
SN1 Reactions in Non-Polar Solvents
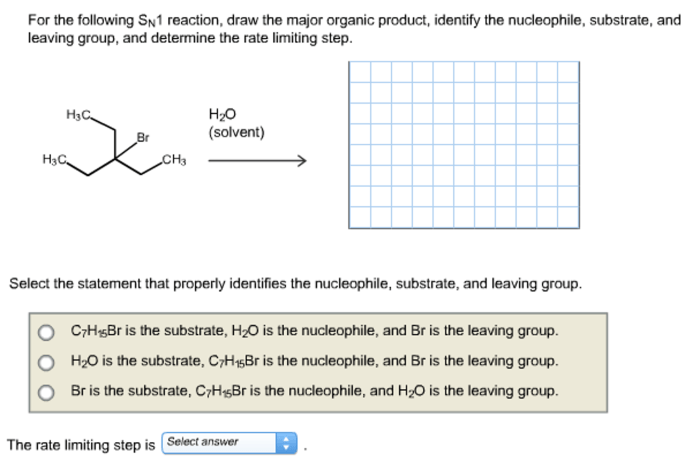
SN1 reactions in non-polar solvents exhibit some unique characteristics. In non-polar solvents, the ions involved in the reaction are not solvated, which makes the ionization step more favorable. This results in faster reaction rates and a higher yield of carbocation intermediates.
Additionally, the absence of solvent stabilization of the carbocation intermediate makes it more reactive, which can lead to a wider range of products.
FAQs: Draw The Major Organic Product Of The Sn1 Reaction
What is the key difference between SN1 and SN2 reactions?
SN1 reactions proceed through a carbocation intermediate, while SN2 reactions occur in a single step with inversion of configuration.
How does solvent polarity affect SN1 reactions?
Polar solvents stabilize the carbocation intermediate, leading to faster reaction rates and increased selectivity.
What factors influence the regioselectivity of SN1 reactions?
The structure of the substrate and the stability of the carbocation intermediate determine the regiochemistry of the reaction.