This dipeptide is designated as a vital component in biological systems, playing a crucial role in various physiological processes. Its unique chemical structure and properties make it a subject of extensive research and applications.
Dipeptides, composed of two amino acids linked by a peptide bond, are essential for protein synthesis and serve as precursors for hormones, neurotransmitters, and other bioactive molecules.
Introduction
A dipeptide is a molecule composed of two amino acids linked by a peptide bond. Dipeptides are the simplest form of proteins and are found in a variety of biological systems. They play important roles in many physiological processes, including protein synthesis, hormone regulation, and immune function.Dipeptides
are formed when two amino acids are joined together by a dehydration reaction. The amino group of one amino acid reacts with the carboxyl group of another amino acid, releasing a molecule of water and forming a peptide bond. The resulting dipeptide has a free amino group at one end and a free carboxyl group at the other end.Dipeptides
are found in a variety of biological systems, including bacteria, plants, and animals. They are particularly abundant in the gastrointestinal tract, where they are produced by the digestion of proteins. Dipeptides are also found in the blood, where they are transported to various tissues and organs.Dipeptides
play important roles in many physiological processes. They are used as building blocks for the synthesis of proteins. They also act as hormones, regulating a variety of cellular processes. In addition, dipeptides play a role in immune function, helping to protect the body from infection.
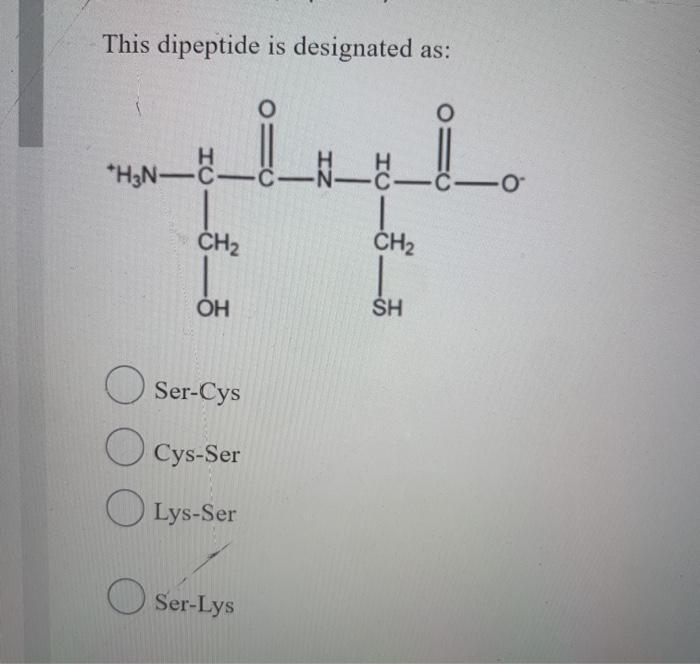
Designation of Dipeptides
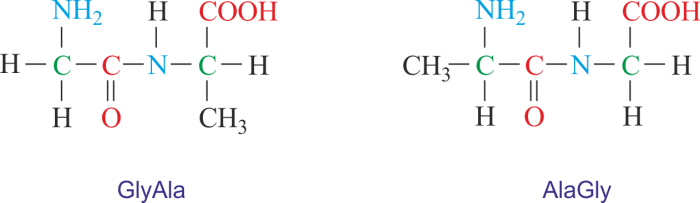
Dipeptides, composed of two amino acids linked by a peptide bond, are designated using various methods. These methods aim to convey information about the amino acid sequence and the nature of the peptide bond.
NomenclatureOne common method is to use the three-letter or one-letter abbreviations of the amino acids followed by the suffix “-yl” for the N-terminal amino acid and “-ine” for the C-terminal amino acid. For example, the dipeptide composed of glycine and alanine can be designated as “Gly-Ala” or “G-A”.
Systematic NomenclatureSystematic nomenclature assigns a unique name to each dipeptide based on the International Union of Pure and Applied Chemistry (IUPAC) recommendations. This method uses the prefixes “amino” and “carboxy” to indicate the N-terminal and C-terminal amino acids, respectively. For instance, the dipeptide Gly-Ala would be named “aminoacetylalanine”.
Other Designations
Other Designations
Other methods of designating dipeptides include using their trivial names, which are often derived from their source or function. For example, the dipeptide composed of aspartic acid and phenylalanine is known as “aspartame”. Additionally, dipeptides can be designated using their molecular weight or their chemical formula.
Chemical Structure and Properties
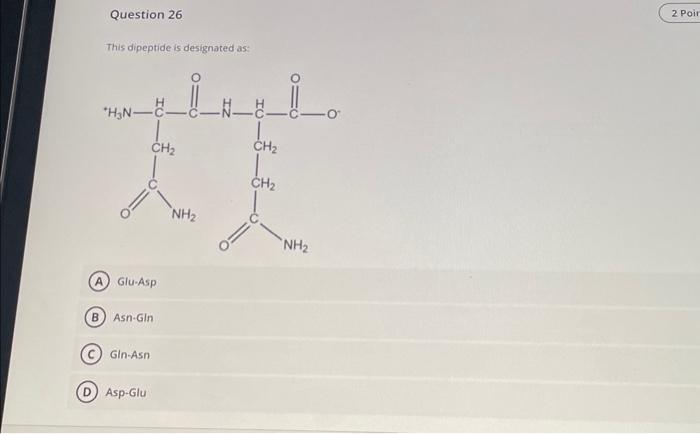
Dipeptides are organic compounds consisting of two amino acids linked by a peptide bond. They are the simplest form of proteins and are essential for many biological processes, such as protein synthesis, hormone regulation, and cell signaling.Dipeptides have a general chemical structure of NH2-CHR1-CO-NH-CHR2-COOH, where R1 and R2 are the side chains of the two amino acids.
The peptide bond is a covalent bond formed between the amino group of one amino acid and the carboxyl group of the other amino acid.The physical and chemical properties of dipeptides depend on the nature of the two amino acids that make them up.
However, some general properties include:
Solubility
Dipeptides are generally soluble in water and other polar solvents. This is due to the presence of polar groups, such as the amino and carboxyl groups, in their structure.
Acidity and Basicity
Dipeptides can act as both acids and bases. The amino group can donate a proton, while the carboxyl group can accept a proton. The acidity and basicity of a dipeptide depend on the pKa values of the two amino acids that make it up.
Reactivity
Dipeptides can undergo a variety of chemical reactions, including hydrolysis, oxidation, and reduction. The reactivity of a dipeptide depends on the nature of the two amino acids that make it up.
Biological Functions: This Dipeptide Is Designated As
Dipeptides play crucial roles in various physiological processes within living organisms. They serve as precursors for protein synthesis, regulate enzymatic activities, and participate in signaling pathways.
Protein Synthesis
Dipeptides act as building blocks for protein synthesis. They are joined together by peptide bonds to form polypeptide chains, which eventually fold into functional proteins.
Enzyme Regulation, This dipeptide is designated as
Dipeptides can modulate the activity of enzymes. For instance, the dipeptide glutathione (GSH) is a potent antioxidant that regulates the activity of enzymes involved in detoxification and redox reactions.
Signaling Pathways
Dipeptides can act as signaling molecules in various cellular processes. For example, the dipeptide carnosine has been shown to have neuroprotective effects and may play a role in regulating inflammation.
Biosynthesis and Metabolism
Dipeptides are synthesized by the ribosome during protein synthesis. The ribosome reads the mRNA sequence and assembles the corresponding amino acids into a polypeptide chain. The first amino acid in the polypeptide chain is typically methionine, which is then removed by a methionine aminopeptidase.
The remaining amino acids are linked together by peptide bonds.Dipeptides are metabolized by a variety of enzymes, including dipeptidases, which break down dipeptides into individual amino acids. Dipeptidases are found in the cytoplasm, mitochondria, and lysosomes. The amino acids released by dipeptidases can be used for protein synthesis, energy production, or other metabolic processes.
Biosynthesis
The biosynthesis of dipeptides involves the following steps:
- The ribosome binds to the mRNA and begins to read the sequence.
- The ribosome recruits the appropriate aminoacyl-tRNA molecules.
- The aminoacyl-tRNA molecules are added to the growing polypeptide chain.
- The ribosome moves along the mRNA, and the process is repeated.
Metabolism
The metabolism of dipeptides involves the following steps:
- Dipeptides are transported into the cell by a variety of transporters.
- Dipeptides are broken down into individual amino acids by dipeptidases.
- The amino acids released by dipeptidases can be used for protein synthesis, energy production, or other metabolic processes.
Applications
Dipeptides have gained significant importance in various fields due to their unique properties and versatility.
In the pharmaceutical industry, dipeptides are used as building blocks for the synthesis of complex peptides and proteins. They are also employed as active pharmaceutical ingredients (APIs) in drugs targeting a wide range of therapeutic areas, including cardiovascular diseases, cancer, and metabolic disorders.
Food and Beverage Industry
Dipeptides play a crucial role in the food and beverage industry as flavor enhancers, sweeteners, and nutritional supplements. They are used in the production of infant formula, sports drinks, and fortified foods.
Cosmetics and Personal Care
Dipeptides are increasingly used in cosmetics and personal care products for their skin-conditioning and anti-aging properties. They are incorporated into moisturizers, serums, and hair care products.
Agriculture
In agriculture, dipeptides are employed as plant growth regulators, promoting root development and enhancing crop yield. They are also used as biopesticides, providing a natural and eco-friendly alternative to chemical pesticides.
Examples of Dipeptide-Based Products
- Aspartame: A dipeptide sweetener used in diet sodas and other low-calorie food products.
- Carnosine: A dipeptide found in muscle tissue that acts as an antioxidant and anti-inflammatory agent.
- Glutathione: A tripeptide that plays a vital role in cellular detoxification and immune function.
Query Resolution
What is the significance of dipeptides in biological systems?
Dipeptides are crucial for protein synthesis and serve as precursors for hormones, neurotransmitters, and other bioactive molecules.
How are dipeptides designated?
Dipeptides can be designated using various methods, including systematic nomenclature, common names, and abbreviations.
What are the key chemical properties of dipeptides?
Dipeptides exhibit amphoteric behavior, meaning they can act as both acids and bases. They also have specific solubility and ionization properties.