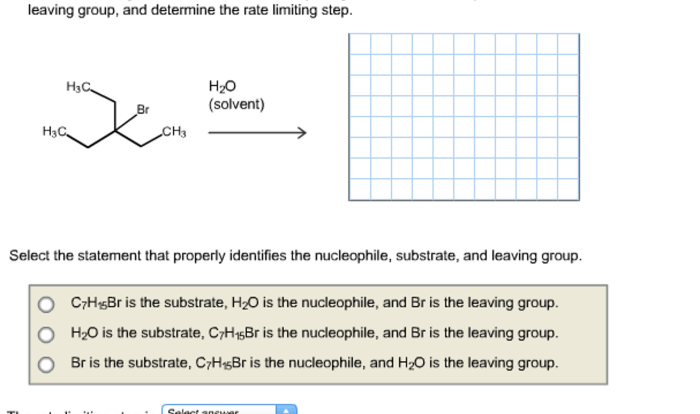
Lewis structure for chloral hydrate, a captivating subject that unravels the intricate molecular architecture of this versatile compound, embarks on a journey of scientific discovery.
Delving into its chemical composition, we unveil the intricate dance of atoms within the chloral hydrate molecule, revealing its structural intricacies and the interplay of electrons that define its unique properties.
Introduction
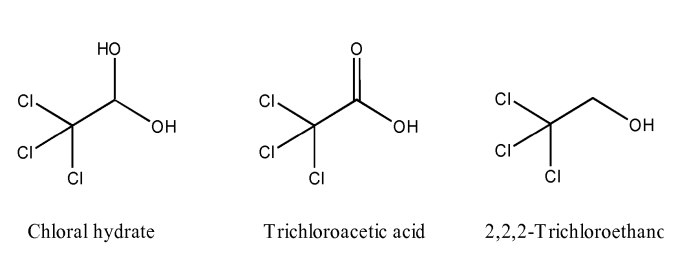
Chloral hydrate, also known as trichloroacetaldehyde monohydrate, is an organic compound with the chemical formula CCl 3CH(OH) 2. It is a white crystalline solid that is soluble in water and alcohol. Chloral hydrate has a sweet, pungent odor and a slightly bitter taste.
Chloral hydrate was first synthesized in 1832 by the German chemist Justus von Liebig. It was initially used as a hypnotic and sedative, but its use has since been largely discontinued due to its potential for abuse and addiction. Chloral hydrate is still used today as a precursor to other chemicals, such as chloroform and trichloroacetic acid.
Uses and Significance
Chloral hydrate has a variety of uses, including:
- As a hypnotic and sedative
- As a precursor to other chemicals, such as chloroform and trichloroacetic acid
- As a reagent in chemical synthesis
Chloral hydrate is also significant because it was one of the first organic compounds to be synthesized in the laboratory. Its synthesis helped to establish the field of organic chemistry and paved the way for the development of many other important chemicals.
Lewis Structure: Lewis Structure For Chloral Hydrate
Drawing the Lewis Structure
The Lewis structure of chloral hydrate, CCl3CH(OH)2, can be drawn by following these steps:
1. Determine the total number of valence electrons
Carbon
4
Chlorine
3 x 7 = 21
Oxygen
2 x 6 = 12
Hydrogen
2 x 1 = 2 Total: 4 + 21 + 12 + 2 = 39 valence electrons
2. Connect the atoms with single bonds to satisfy the octet rule for each atom
Cl-C-C-O-H | | | Cl H H
3. Distribute the remaining electrons as lone pairs
Cl-C-C-O-H | | | Cl H H | | | Cl O O
Central Atom and Hybridization
The central atom in chloral hydrate is carbon. It has four electron groups (three single bonds and one double bond) around it, which indicates sp3 hybridization.
Electron Geometry and Molecular Geometry
The electron geometry around the central carbon atom is tetrahedral. However, due to the presence of three lone pairs on the oxygen atoms, the molecular geometry is distorted and adopts a trigonal pyramidal shape.
Bond Parameters
The bond parameters of chloral hydrate, including bond lengths, bond angles, and bond polarity, provide insights into the molecular structure and interactions within the compound.
Bond Lengths and Bond Angles
Chloral hydrate exhibits specific bond lengths and bond angles due to the hybridization of its constituent atoms. The carbon atom in the carbonyl group (C=O) is sp 2hybridized, resulting in trigonal planar geometry. The bond length between the carbon and oxygen atoms in the carbonyl group is approximately 1.22 Å. The carbon atom in the CCl 3group is also sp 2hybridized, leading to trigonal planar geometry.
The bond lengths between the carbon and chlorine atoms in the CCl 3group are approximately 1.78 Å.
Bond Polarity
The bonds in chloral hydrate exhibit varying degrees of polarity due to the electronegativity differences between the atoms involved. The C=O bond is polar, with the oxygen atom being more electronegative than the carbon atom. This results in a partial negative charge on the oxygen atom and a partial positive charge on the carbon atom.
The C-Cl bonds are also polar, with the chlorine atoms being more electronegative than the carbon atom. This leads to a partial negative charge on the chlorine atoms and a partial positive charge on the carbon atom.
Resonance Structures, Lewis structure for chloral hydrate
Chloral hydrate does not exhibit resonance structures because there are no equivalent Lewis structures with the same number of bonds and electrons.
Molecular Properties
The molecular properties of chloral hydrate are determined by its molecular structure and the interactions between its constituent atoms.
Chloral hydrate is a white, crystalline solid with a melting point of 50.8 °C and a boiling point of 97.6 °C. It is soluble in water, alcohol, and ether.
Solubility
Chloral hydrate is highly soluble in water, with a solubility of 50 g/100 mL at 20 °C. This high solubility is due to the formation of hydrogen bonds between the water molecules and the hydroxyl group of chloral hydrate.
Melting Point
The melting point of chloral hydrate is 50.8 °C. This relatively low melting point is due to the weak intermolecular forces between the chloral hydrate molecules.
Boiling Point
The boiling point of chloral hydrate is 97.6 °C. This relatively low boiling point is due to the small size and low molecular weight of chloral hydrate.
Reactivity
Chloral hydrate is a reactive compound that can undergo a variety of reactions, including:
- Hydrolysis: Chloral hydrate can hydrolyze to form chloral and water.
- Oxidation: Chloral hydrate can be oxidized to form chloroform and chloric acid.
- Reduction: Chloral hydrate can be reduced to form trichloroethanol.
Potential Applications
Chloral hydrate has a variety of potential applications, including:
- As a sedative and hypnotic
- As an anticonvulsant
- As a topical antiseptic
- As a solvent
Comparison with Other Molecules
Chloral hydrate, with its unique structural features, exhibits similarities and differences when compared to other molecules. Understanding these comparisons provides insights into its chemical behavior and properties.
Comparison with Chloral
Chloral, the parent compound of chloral hydrate, shares a similar molecular structure. Both molecules consist of a central carbon atom bonded to three chlorine atoms and an oxygen atom. However, chloral hydrate has an additional hydroxyl group (-OH) attached to the central carbon, which distinguishes it from chloral.
This structural difference significantly alters their properties. Chloral is a highly reactive liquid, while chloral hydrate is a solid at room temperature. The hydroxyl group in chloral hydrate forms hydrogen bonds with water molecules, leading to its solubility in water and higher stability.
Comparison with Alcohols
Chloral hydrate also exhibits similarities with alcohols, as it contains a hydroxyl group. However, the presence of the three chlorine atoms attached to the central carbon introduces unique characteristics.
Unlike simple alcohols, chloral hydrate has a much lower acidity. The electron-withdrawing effect of the chlorine atoms reduces the acidity of the hydroxyl group, making it less likely to donate a proton.
Comparison with Hydrates
Chloral hydrate belongs to a class of compounds known as hydrates, which contain water molecules as part of their crystal structure. The hydroxyl group in chloral hydrate forms hydrogen bonds with water molecules, resulting in the formation of a crystalline hydrate.
Compared to other hydrates, chloral hydrate exhibits a relatively low water content. It contains one water molecule per molecule of chloral hydrate, while many other hydrates have higher water contents.
Applications and Implications
Chloral hydrate has various practical applications, particularly in the medical field.
Sedative and Hypnotic
Chloral hydrate acts as a sedative, inducing relaxation and reducing anxiety. It has been traditionally used as a hypnotic to promote sleep, particularly in cases of insomnia. Its sedative effects are attributed to its ability to depress the central nervous system.
Antiseptic
Chloral hydrate possesses antiseptic properties, making it effective in inhibiting the growth of microorganisms. It is commonly used as an ingredient in mouthwashes and throat lozenges to combat oral infections and reduce inflammation.
Other Applications
Beyond its medicinal uses, chloral hydrate has found applications in other fields:
- Textile industry:As a mordant, chloral hydrate helps dyes adhere to fabrics, enhancing their colorfastness.
- Photography:Chloral hydrate is employed as a developer in certain photographic processes.
FAQ Corner
What is the hybridization of the central carbon atom in chloral hydrate?
sp3
How many resonance structures does chloral hydrate have?
3
What is the polarity of the C-Cl bond in chloral hydrate?
Polar covalent